Reactive spin state dependent enantiospecific photocyclization of axially chiral α-substituted acrylanilides†
Abstract
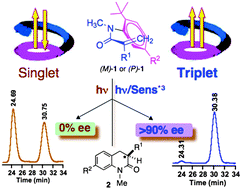
The enantiomeric ratio (e.r.) in the 3,4-dihydroquinolin-2-one photoproduct during 6π-photocyclization of α-substituted axially chiral ortho-tert-butyl-acrylanilides depends on the nature of the reactive spin state (singlet or triplet), where the singlet-spin state reactivity gives a racemic mixture and the triplet reactivity gives an e.r. value >95 ∶ 5.

 Please wait while we load your content...
Please wait while we load your content...