Agonist responses of (R)- and (S)-3-fluoro-γ-aminobutyric acids suggest an enantiomeric fold for GABA binding to GABAC receptors†
Abstract
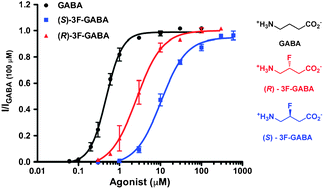
The enantiomers of 3F-GABA were evaluated on GABAC receptors. Both enantiomers were
- This article is part of the themed collection: RACI100: Celebrating Australian Chemistry

 Please wait while we load your content...
Please wait while we load your content...