Hypervalent phenyl-λ3-iodane-mediated para-selective aromatic fluorination of 3-phenylpropyl ethers†
Abstract
Exposure of 3-phenylpropyl ethers to an activated iodosylbenzene monomer·18-crown-6 complex [PhI(OH)BF4·18C6] in the presence of BF3–Et2O and
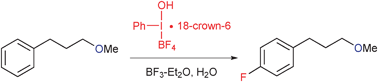

 Please wait while we load your content...
Please wait while we load your content...