Self-assembly of amphiphilic calixarenes and resorcinarenes in water
Abstract
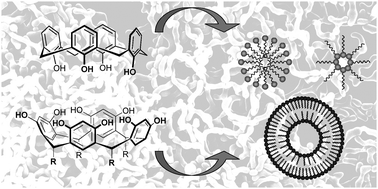
The calixarenes and resorcinarenes are macrocyclic phenolic molecules that can be modified “à façon” and a wide range of chemical modification strategies have been published over the last 30 years. Because of their remarkable structural properties and their relative ease of chemical modification, they represent excellent and highly versatile bases to design complex building blocks capable of

 Please wait while we load your content...
Please wait while we load your content...