Highly enantioselective synthesis of 1,3-bis(hydroxymethyl)-2-oxindoles from unprotected oxindoles and formalin using a chiral NdIII complex†
Abstract
Highly enantioselective synthesis of 1,3-bis(hydroxymethyl)-2-oxindoles was established through chiral
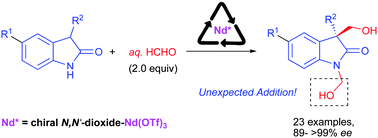

 Please wait while we load your content...
Please wait while we load your content...