The organocatalytic three-step total synthesis of (+)-frondosin B†
Abstract
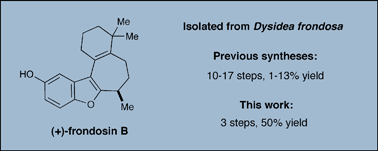
The frondosins are a family of marine sesquiterpenes isolated from the sponge Dysidea frondosa that exhibit biological activities ranging from anti-inflammatory properties to potential application in anticancer and HIV therapy. Herein, a concise enantioselective total synthesis of

 Please wait while we load your content...
Please wait while we load your content...