Exploring the effects of solvent polarity on the rate of Förster-type electronic energy transfer in a closely-spaced molecular dyad†
Abstract
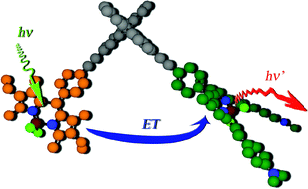
A rigid molecular dyad, comprising two disparate
- This article is part of the themed collection: In honour of Jan Verhoeven

 Please wait while we load your content...
Please wait while we load your content...