Challenges associated with the synthesis of unusual o-carboxamido stilbenes by the Heck protocol: Intriguing substituent effects, their toxicological and chemopreventive implications†
Abstract
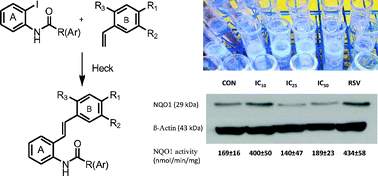
The syntheses of fourteen unusual o-carboxamido stilbenes by the Heck protocol revealed surprising complexity related to intriguing substituent effects with mechanistic implications. The unexpected cytotoxic and chemopreventive properties also seem to be substituent dependent. For example, although

 Please wait while we load your content...
Please wait while we load your content...