Design and synthesis of nonpeptidic, small molecule inhibitors for the Mycobacterium tuberculosisprotein tyrosine phosphatase PtpB†‡
Abstract
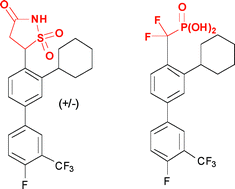
The design and synthesis of new
- This article is part of the themed collection: Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...