Flexible and enantioselective access to jaspine B and biologically active chain-modified analogues thereof†
Abstract
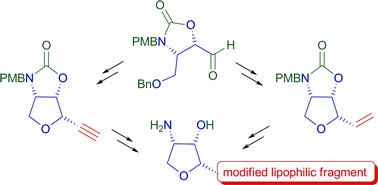
Whereas the all-cis tetrahydrofuran framework of the cytotoxic anhydrophytosphingosine jaspine B is considered as a relevant pharmacophore, little is known about the influence of the aliphatic chain of this amphiphilic molecule on its activity. We developed a synthetic strategy allowing flexible introduction of various lipophilic fragments in the jaspine's skeleton. The route was validated with two distinct approaches to jaspine B. Five chain-modified analogues were also prepared. Biological evaluation of these derivatives demonstrated a good correlation between their cytotoxicity and their capacity to inhibit conversion of ceramide into sphingomyelin in melanoma cells. A series of potent and selective inhibitors of sphingomyelin production was thus identified. Furthermore, the good overall potency of an ω-aminated analogue allowed us to dissociate of the pharmacological action of jaspine B from its amphiphilic nature.

 Please wait while we load your content...
Please wait while we load your content...