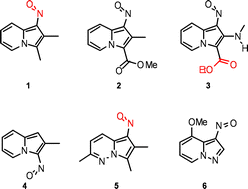
Conformational equilibria and barriers to rotation in some novel nitroso derivatives of indolizines and 3- and 5-azaindolizines – an NMR and molecular modeling study†
Abstract
Conformational equilibria in novel C-nitroso derivatives of indolizines and 3- and 5-azaindolizines have been studied by
 Please wait while we load your content...
Please wait while we load your content...