An efficient synthesis of D-2′-deoxy-2′,2′-difluoro-4′-dihydro-4′-thionucleosides is described. The conformations of D-2′-deoxy-2′,2′-difluoro-4′-dihydro-4′-thiouridine were studied by X-ray crystallography, NMR spectroscopy and molecular modeling in an attempt to explore the roles of the two gem-difluorine atoms in the puckering preferences of the thiosugar ring. No matter which conformation (south or north) the thiosugar adopts, there is always one fluorine in a pseudoaxial position, with the other in a pseudoequitorial position and thus the strong antiperiplanar (ap) effects from C–H and C–C σ-bonds to σ*C–F are equal to each other in these two conformers. Therefore, the other weak effects, such as dipole–dipole interactions and electrostatic attractions, become more important for determining the overall conformation of the sugar ring. Based on the results of NMR spectroscopy, high-level quantum computations and molecular dynamic simulations were performed to study the preferred pucker of the thiosugar ring in solution. Our results showed that the strong antiperiplanar preference of C–H and C–C σ-bonds to σ*C–F and σ*C–O seemed to be responsible for the favored S-conformation in solution, and the weak electrostatic attractions between δ+C2–Fβδ− and δ+H6–C6δ- may stabilize the preferred structure further, and keep the base moiety in a high anti-rotamer population in solution. In contrast, the packing forces (hydrogen bond OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, dipole–dipole interaction C–F⋯C
C, dipole–dipole interaction C–F⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the solid state compensated the energetic disadvantage of the relatively less stable N-conformation, and drove the thiouridine to crystallize in the N-conformation. These results, along with the earlier empirical rules regarding proton chemical shifts in carbohydrates and nucleosides, were used to propose a method based on proton chemical shifts for the analysis of the N⇌S equilibrium of the fluorinated sugar ring.
O) in the solid state compensated the energetic disadvantage of the relatively less stable N-conformation, and drove the thiouridine to crystallize in the N-conformation. These results, along with the earlier empirical rules regarding proton chemical shifts in carbohydrates and nucleosides, were used to propose a method based on proton chemical shifts for the analysis of the N⇌S equilibrium of the fluorinated sugar ring.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, dipole–dipole interaction C–F⋯C
C, dipole–dipole interaction C–F⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the solid state compensated the energetic disadvantage of the relatively less stable N-conformation, and drove the
O) in the solid state compensated the energetic disadvantage of the relatively less stable N-conformation, and drove the 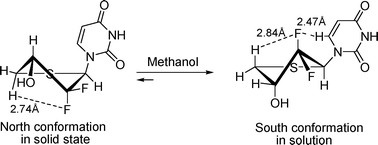

 Please wait while we load your content...
Please wait while we load your content...