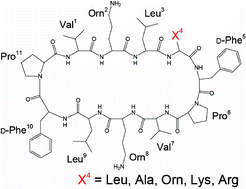
Antimicrobially active cycloundecapeptides related to gramicidin S, cyclo(-Val1-Orn2-Leu3-X4-D-Phe5-Pro6-Val7-Orn8- Leu9-D-Phe10-Pro11-) (X= Leu (1), Ala (2), Orn (3), Lys (4) and Arg (5)), were synthesized. From the CD and NMR studies, 1–5 possess antiparallel β-sheet conformation linked by a type II’β-turn around D-Phe10-Pro11 and a novel turn structure around X4-D-Phe5-Pro6 sequence with cisD-Phe-Pro peptide bond. The structural modifications at position 4 of 1–5 are beneficial to identification of novel antibiotic candidates without hemolytic activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...