Impact of the colloidal state on the oriented attachment growth mechanism
Abstract
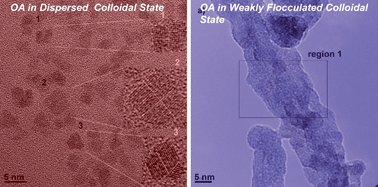
In the last five years, several excellent reviews about oriented attachment (OA) have evidenced the advances achieved in this research area, detailing the
- This article is part of the themed collection: Crystallization and Formation Mechanisms of Nanostructures

 Please wait while we load your content...
Please wait while we load your content...