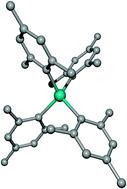
Heterobimetallic compounds with complex cations of the very electropositive alkaline earth metals (Ae) and organic tetrahedral anions of trivalent elements (M) form solvent-separated ions. Depending on the metals, they can be prepared by addition of carbanions or amides to MR3, via reduction of VMes4 with the alkaline earth metals and by transmetallation of VMes3. For comparison reasons selected alkali metal derivatives are also included in this study. Average structural parameters of the boranates [Ca(thf)6][BPh4]2 (1) and [CaI(thf)5][BPh4] (2), the alanates [Li(thf)2(tmeda)][AlPh3(tmp)] (3), [(thf)2K(N-carbazolyl)2AlMes2] (4), and [Sr(thf)7][AlPh4]2 (5), of the gallate [Ca(dme)4][GaEt3(N-carbazolyl)]2 (6), and of the vanadates [Li(thf)4][VMes4] (7), [CaI(thf)5][VMes4] (8), [Ca(thf)6][VMes4]2 (9), and [Sr(thf)6][VMes4]2 (10), are discussed. All these complexes contain complex cations of the type [(L)nM]+, and tetrahedral anions of the type [ER4]−. Only 4 crystallizes as a contact ion pair because the soft K+ cation shows no preference for hard bases such as ethers or for soft arene π-systems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...