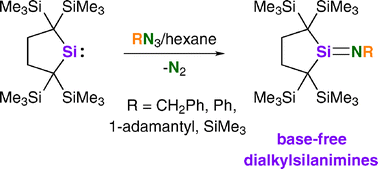
Abstract
Four isolable dialkylsilanimines RH2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR (R = CH2Ph (5a), Ph (5b),
NR (R = CH2Ph (5a), Ph (5b), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R structure, while 5c and 5d have an almost linear structure. Distinct π(Si
N–R structure, while 5c and 5d have an almost linear structure. Distinct π(Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)
N) →
→ π*(Si
π*(Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and
N) and  →
→ π*(Si
π*(Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) transition bands of
N) transition bands of
- This article is part of the themed collection: Main group elements

 Please wait while we load your content...
Please wait while we load your content...