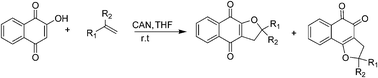
We report herein, the synthesis and antifungal activity of substituted α- and β-dihydrofuran naphthoquinones. These compounds were prepared from readily available lawsone and olefins in the presence of cerium(IV) ammonium nitrate (CAN) and were then evaluated against the following six strains of Candida (C.): C. albicans, C. krusei, C. parapsilosis, C. kefyr, C. tropicalis and C. dubliniensis. In addition to exhibiting low cytotoxicity, the furan naphthoquinones proved to be active against these fungi, indicating that they are important scaffolds and potential novel antifungal agents.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...