Structure of the EGF receptor transactivation circuit integrates multiple signals with cell context†
Abstract
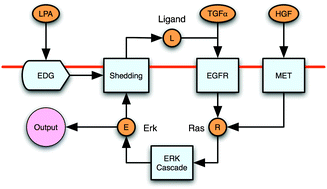
Transactivation of the epidermal growth factor receptor (EGFR) is thought to be a process by which a variety of cellular inputs can be integrated into a single signaling pathway through either stimulated proteolysis (shedding) of membrane-anchored EGFR

 Please wait while we load your content...
Please wait while we load your content...