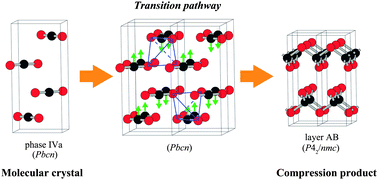
When first principles calculations are systematically made with high numerical accuracy, they can be successfully combined with statistical thermodynamics in order not only to “reproduce” the experimental data but also to “predict” as-yet-unknown structures and properties. Lattice dynamics calculations can be used to compute the temperature dependence of thermodynamical quantities such as free energy. The products and pathways of phase transitions can also be analyzed on the basis of the information of imaginary phonon modes. Cluster expansion technique can be used to take account the statistical thermodynamics of crystalline solutions. Ground state structures within a given lattice model as well as the thermostatistical information of crystalline solutions or disordered compounds with temperature can be investigated. Such approaches require a large set of first principles calculations, which has become possible only recently. In this article a few examples using the combined methods are given. They include ZrO2, Ga2O3, Bi2O3, CO2, spinel oxides, and SnO2−x.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...