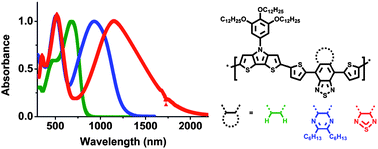
A series of highly soluble donor–acceptor (D–A) copolymers containing N-(3,4,5-tri-n-decyloxyphenyl)-dithieno[3,2-b:2′,3′-d]pyrrole (DTP) or N-(2-decyltetradecyl)-dithieno[3,2-b:2′,3′-d]pyrrole (DTP′) as donor and three different acceptors, 4,7-dithien-2-yl-[2,1,3]-benzothiadiazole, 4,9-dithien-2-yl-6,7-di-n-hexyl-[1,2,5]thiadiazolo[3,4-g]quinoxaline and 4,8-dithien-2-yl-2λ4δ2-benzo[1,2-c;4,5-c′]bis[1,2,5]thiadiazole (BThX, X = BTD, TQHx2, BBT, respectively) were synthesized by Stille coupling polymerizations. The optical and electrochemical properties of these copolymers were investigated, along with their use in field-effect transistors and photovoltaic devices. The band gaps (eV) estimated from UV-vis-NIR spectra and electrochemical measurements of the copolymers varied from ca. 1.5–0.5 eV, and were consistent with quantum-chemical estimates extrapolated using density functional theory. Oxidative and reductive spectroelectrochemistry of the copolymers indicated they can be both p-doped and n-doped, and three to four differently colored redox states of the polymers can be accessed through electrochemical oxidation or reduction. The DTP-BThBTD and DTP-BThTQHx2 copolymers exhibited average field-effect hole mobilities of 1.2 × 10−4 and 2.2 × 10−3 cm2/(Vs), respectively. DTP-BThBBT exhibited ambipolar field-effect characteristics and showed hole and electron mobilities of 1.2 × 10−3 and 5.8 × 10−4 cm2/(Vs), respectively. Bulk heterojunction photovoltaic devices made from blends of the copolymers with 3′-phenyl-3′H-cyclopropa[1,9](C60-Ih)[5,6]fullerene-3′-butanoic acid methyl ester (PCBM) (1:3 weight ratio) exhibited average power conversion efficiencies as high as 1.3% under simulated irradiance of 75 mW/cm2.

 Please wait while we load your content...
Please wait while we load your content...