Solvent-free solid acid-catalyzed nucleophilic substitution of propargylic alcohols: a green approach for the synthesis of 1,4-diynes†
Abstract
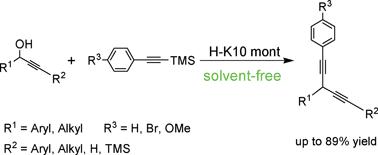
Acid-treated K10 montmorillonite (H-K10 mont) exhibited an outstanding catalytic activity in the nucleophilic substitution of propargylic

 Please wait while we load your content...
Please wait while we load your content...