Reversible zwitterionic liquids, the reaction of alkanol guanidines, alkanol amidines, and diamines with CO2
Abstract
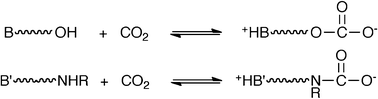
Alkanolamidines, alkanolguanidines and diamines each react with CO2 to form reversible zwitterionic liquids. CO2 is chemically bound to the alcohol on alkanolamidines and alkanolguanidines as zwitterionic alkylcarbonates, while CO2 is chemically bound on diamines as zwitterionic carbamates. All three classes of zwitterionic liquids could be reverted to their non-ionic forms by thermally stripping the CO2 at temperatures near 50 °C, while one derivative was found to irreversibly form a cyclic

 Please wait while we load your content...
Please wait while we load your content...