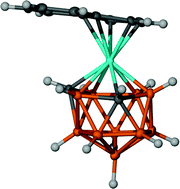
13-vertex indenyl cobaltacarboranes with 4,1,6-, 4,1,10- and 4,1,2-CoC2B10 architectures have been synthesised by reduction of the corresponding closo carborane and metallation with an {(η-C9H7)Co} fragment. Variants of the 4,1,6-isomer were prepared with no, one and two methyl groups on cage C atoms, whilst 4,1,2-species were obtained both with two methyl groups and a trimethylene tether on the cage C atoms. Thermolysis of the 4,1,6-isomers yielded the corresponding 4,1,8-isomers, which in turn were converted to 4,1,12-isomers by thermolysis at higher temperatures. Alternatively relatively mild heating of the 4,1,10-isomer led to the 4,1,12-isomer directly. Products were characterised by mass spectrometry, 1H and 11B NMR spectroscopies and, in most cases, elemental analysis, and nine compounds were studied crystallographically. The 4,1,6-, 4,1,8-, 4,1,10- and 4,1,12- species have docosahedral cages whilst the 4,1,2-species are henicosahedral. In the structural studies attention focused on the orientation of the indenyl ligand with respect to the carborane ligand since this affords experimental information on the metal-cage bonding through the structural indenyl effect. There is a general tendency for the indenyl ligand to adopt orientations in which the ring junction C atoms lie trans to cage B atoms. In cases where the orientation is not compromised by the presence of a non-H substituent on the face of the carborane there is generally good agreement between the experimental orientation and that computed by DFT calculations for the related naphthalene ferracarboranes (η-C10H8)FeC2B10H12. The presence of C-methyl substituents in the indenyl cobaltacarboranes tends to override this preference except in the case of 1,6-Me2-4-(η-C9H7)-4,1,6-closo-CoC2B10H10 where the indenyl ligand instead is forced to incline away from the cage methyl groups. In DCM solution the 4,1,6-, 4,1,8-, 4,1,10- and 4,1,12- isomers of (η-C9H7)CoC2B10H12 exhibit two, stepwise, 1-electron reductions assigned to Co(III)/Co(II)/Co(I) couples at less negative potentials than those of the corresponding Cp compounds. Moreover these reductions are easier for those isomers (4,1,6- and 4,1,10-) in which there are two cage C atoms in the carborane face to which the metal atom is bound. By spectroelectrochemical and EPR measurements it is concluded that the reductions of these indenyl cobaltacarboranes are largely metal-based.

 Please wait while we load your content...
Please wait while we load your content...