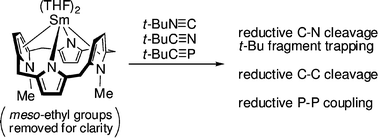
Sm(ii)reduction chemistry of heteroalkynes: stable adducts, reductive coupling, reductive C–C/C–N bond cleavage and trapping of the tert-butyl fragment with bulky nitriles, phosphaalkynes and isonitriles†
Abstract
Reactions of a dimetallated ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PP
PP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-t-Bu)2− featuring a new
C-t-Bu)2− featuring a new ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N
N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N
N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-t-Bu)− complex.
N-t-Bu)− complex.
- This article is part of the themed collection: New horizons in organo-f-block chemistry

 Please wait while we load your content...
Please wait while we load your content...