The acyclic dialkylstannylene [(Me3Si){Me2P(BH3)}CH]2Sn (7) reacts with any of methyl iodide, neopentyl iodide or benzyl bromide to yield the corresponding oxidative addition products [(Me3Si){Me2P(BH3)}CH]2Sn(Me)(I) (8), [(Me3Si){Me2P(BH3)}CH]2Sn(CH2CMe3)(I) (9), and [(Me3Si){Me2P(BH3)}CH]2Sn(CH2Ph)(Br) (10), respectively. The crystal structures of 8, 9, and 10 reveal that there are no close B–H⋯Sn contacts. In addition, 7 reacts with benzil or elemental sulfur to yield [{Me2P(BH3)}(Me3Si)CH]2Sn(OCPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPhO) (11) and [{Me2P(BH3)}(Me3Si)CH]2Sn(S) (12), respectively, as confirmed by multinuclear NMR spectroscopy and elemental analysis.
CPhO) (11) and [{Me2P(BH3)}(Me3Si)CH]2Sn(S) (12), respectively, as confirmed by multinuclear NMR spectroscopy and elemental analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPhO) (11) and [{Me2P(BH3)}(Me3Si)CH]2Sn(S) (12), respectively, as confirmed by multinuclear
CPhO) (11) and [{Me2P(BH3)}(Me3Si)CH]2Sn(S) (12), respectively, as confirmed by multinuclear 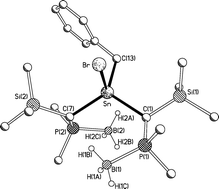

 Please wait while we load your content...
Please wait while we load your content...