Syntheses and characterization of bis(trifluoromethyl)phosphino naphthalenes and acenaphthenes†
Abstract
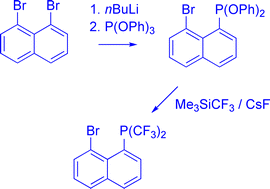
Syntheses of heteroleptic 1,8-bis(phosphino)naphthalenes and 5,6-bis(phosphino)acenaphthenes were attempted using several synthetic strategies. Reaction of aryllithium with

 Please wait while we load your content...
Please wait while we load your content...