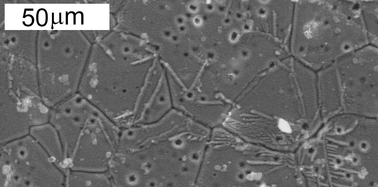
The high temperature demixing/recombination phenomenon previously observed in Ca-substituted La2Mo2O9 oxide ion conductors [A. Selmi et al., Solid State Ionics, 2006, 177, 3051; Eur. J. Inorg. Chem., 2008, 1813] has been visualised using scanning electron microscopy and EDX analysis. The demixed state appears as CaMoO4 straight solid streams erupted from pores within LAMOX grains. The thermal stability study is extended to other alkali and alkaline-earth substituted LAMOX compounds, all of which are shown, in temperature-controlled X-ray diffractograms, to present similar demixing/recombination processes. The most spectacular effect is observed in La1.88K0.12Mo0.6W1.4O8.88 where demixing takes the form of a total decomposition, before full recombination at a higher temperature. Such a phenomenon is interpreted as originating from temperature-dependent solid solution limits with higher substitutional ranges at higher temperatures. It results in the metastabilisation of pure phases by quenching (or rapid cooling), whereas the stable state is demixed, as shown on slowly cooled samples.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...