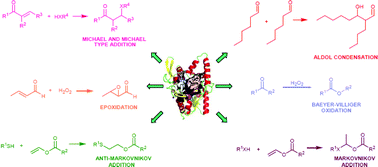
During the last three decades the use of hydrolases for the catalysis of environmentally friendly organic processes under mild reaction conditions has been well documented. Hydrolases have shown themselves to be ideal tools for the acceleration of synthetic transformations because of their high stability, catalytic efficiency, commercial availability and broad substrate specificity in a wide spectrum of biocatalyzed processes. In recent years, novel examples have appeared related to non-conventional reactions catalyzed by hydrolytic enzymes. Amongst these, lipases and acylases have gained much attention as promiscuous biocatalysts showing good levels of reactivity in C–C bond formation, C–heteroatom bond formation, oxidative processes, and novel hydrolytic reactions. This critical review covers recent investigation in the field of catalytic promiscuity, and highlights the most surprising and uncommon activities that this class of enzymes shows in organic synthetic transformations (111 references).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...