In situ click chemistry: probing the binding landscapes of biological molecules†
Abstract
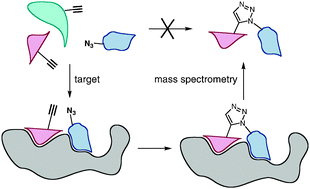
Combinatorial approaches to the discovery of new functional molecules are well established among chemists and biologists, inspired in large measure by the modular composition of many systems and molecules in Nature. Many approaches rely on the synthesis and testing of individual members of a candidate combinatorial library, but attention has also been paid to techniques that allow the target to self-assemble its own binding agents. These fragment-based methods, grouped under the general heading of target-guided synthesis (TGS), show great promise in lead discovery applications. In this tutorial review, we review the use of the
- This article is part of the themed collection: Click chemistry: Function follows form

 Please wait while we load your content...
Please wait while we load your content...