The ground state potential energy surfaces (PESs) of MH2n+ (M = Li, Be, Na, Mg, K, Ca; n = 1, 2) have been investigated using relativistically corrected, coupled-cluster (CC) and multi-reference configuration interaction (MRCI) methods. The PESs for MH2+ (M = Li, Na, K) and MH2n+ (M = Be, Mg, Ca; n = 1, 2) exhibit global minima corresponding to C2v symmetry equilibrium structures, with local minima for D∞h and C∞v symmetry states. Conversely, the ground state PESs of LiH22+, NaH22+ and KH22+ are repulsive. In all cases, the D∞h states resulting from the insertion of Mn+ into the H2 moiety were significantly higher in energy than the co-linear C2v states. It is generally assumed a priori that these species are the result of the interaction between the metal ion charge state and the quadrupole moment of the H2 moiety. However, analysis of the functional Δ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα(R(Mn+–H2)) =
αα(R(Mn+–H2)) = ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα(MH2n+) −
αα(MH2n+) − ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα(Mn+) (which is effectively the difference between traceless quadrupole moments (
αα(Mn+) (which is effectively the difference between traceless quadrupole moments (![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα) of MH2n+ and the isolated Mn+ ion computed using MRCI) as a function of Mn+–H2 distance demonstrates that a local maximum in Δ
αα) of MH2n+ and the isolated Mn+ ion computed using MRCI) as a function of Mn+–H2 distance demonstrates that a local maximum in Δ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα along the molecular C2 axis is necessary for the formation of a thermodynamically stable complex. It is concluded that the topology of Δ
αα along the molecular C2 axis is necessary for the formation of a thermodynamically stable complex. It is concluded that the topology of Δ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα provides a convenient indicator of the stability of such molecular ion–quadrupole complexes.
αα provides a convenient indicator of the stability of such molecular ion–quadrupole complexes.
![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα(R(Mn+
αα(R(Mn+![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα(MH2n+) −
αα(MH2n+) − ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα(Mn+)
αα(Mn+)![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα) of MH2n+ and the isolated
αα) of MH2n+ and the isolated ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα along the molecular C2 axis is necessary for the formation of a thermodynamically stable complex. It is concluded that the topology of Δ
αα along the molecular C2 axis is necessary for the formation of a thermodynamically stable complex. It is concluded that the topology of Δ![[capital Theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e10a.gif) αα provides a convenient
αα provides a convenient 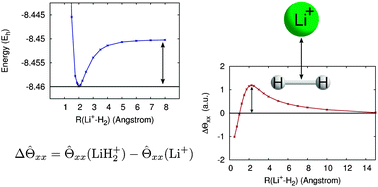

 Please wait while we load your content...
Please wait while we load your content...