Fuel decomposition and benzene formation processes in a premixed, laminar, low-pressure, fuel-rich flame of 1-hexene (C6H12, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH2–CH2–CH3) are investigated by comparing quantitative mole fraction profiles of flame species with kinetic modeling results. The premixed flame, which is stabilized on a flat-flame burner under a reduced pressure of 30 Torr (= 40 mbar), is analyzed by flame-sampling molecular-beam time-of-flight mass spectrometry which uses photoionization by tunable vacuum-ultraviolet synchrotron radiation. The temperature profile of the flame is measured by OH laser-induced fluorescence. The model calculations include the latest rate coefficients for 1-hexene decomposition (J. H. Kiefer et al., J. Phys. Chem. A, 2009, 113, 13570) and for the propargyl (C3H3) + allyl (a-C3H5) reaction (J. A. Miller et al., J. Phys. Chem. A, 2010, 114, 4881). The predicted mole fractions as a function of distance from the burner are acceptable and often even in very good agreement with the experimentally observed profiles, thus allowing an assessment of the importance of various fuel decomposition reactions and benzene formation routes. The results clearly indicate that in contrast to the normal reactions of fuel destruction by radical attack, 1-hexene is destroyed mainly by decomposition via unimolecular dissociation forming allyl (a-C3H5) and n-propyl (n-C3H7). Minor fuel-consumption pathways include H-abstraction reactions producing various isomeric C6H11 radicals with subsequent β-scissions into C2, C3, and C4 intermediates. The reaction path analysis also highlights a significant contribution through the propargyl (C3H3) + allyl (a-C3H5) reaction to the formation of benzene. In this flame, benzene is dominantly formed through H-assisted isomerization of fulvene, which itself is almost exclusively produced by the C3H3 + a-C3H5 reaction.
CH–CH2–CH2–CH2–CH3) are investigated by comparing quantitative mole fraction profiles of flame species with kinetic modeling results. The premixed flame, which is stabilized on a flat-flame burner under a reduced pressure of 30 Torr (= 40 mbar), is analyzed by flame-sampling molecular-beam time-of-flight mass spectrometry which uses photoionization by tunable vacuum-ultraviolet synchrotron radiation. The temperature profile of the flame is measured by OH laser-induced fluorescence. The model calculations include the latest rate coefficients for 1-hexene decomposition (J. H. Kiefer et al., J. Phys. Chem. A, 2009, 113, 13570) and for the propargyl (C3H3) + allyl (a-C3H5) reaction (J. A. Miller et al., J. Phys. Chem. A, 2010, 114, 4881). The predicted mole fractions as a function of distance from the burner are acceptable and often even in very good agreement with the experimentally observed profiles, thus allowing an assessment of the importance of various fuel decomposition reactions and benzene formation routes. The results clearly indicate that in contrast to the normal reactions of fuel destruction by radical attack, 1-hexene is destroyed mainly by decomposition via unimolecular dissociation forming allyl (a-C3H5) and n-propyl (n-C3H7). Minor fuel-consumption pathways include H-abstraction reactions producing various isomeric C6H11 radicals with subsequent β-scissions into C2, C3, and C4 intermediates. The reaction path analysis also highlights a significant contribution through the propargyl (C3H3) + allyl (a-C3H5) reaction to the formation of benzene. In this flame, benzene is dominantly formed through H-assisted isomerization of fulvene, which itself is almost exclusively produced by the C3H3 + a-C3H5 reaction.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH2–CH2–CH3) are investigated by comparing quantitative mole fraction profiles of flame species with kinetic modeling results. The premixed flame, which is stabilized on a flat-flame burner under a reduced pressure of 30 Torr (= 40 mbar), is analyzed by flame-sampling molecular-beam
CH–CH2–CH2–CH2–CH3) are investigated by comparing quantitative mole fraction profiles of flame species with kinetic modeling results. The premixed flame, which is stabilized on a flat-flame burner under a reduced pressure of 30 Torr (= 40 mbar), is analyzed by flame-sampling molecular-beam 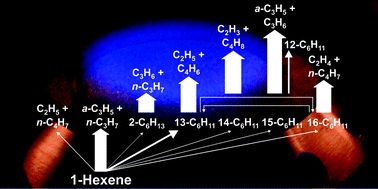

 Please wait while we load your content...
Please wait while we load your content...