The conformational landscape of the volatile anesthetic sevoflurane†
Abstract
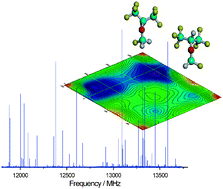
Most of the volatile haloorganic compounds used as anesthetics exhibit a heavy-atom frame large enough to allow for conformational changes. Even in the absence of directed intermolecular interactions, only some or just one of the possible conformations might have an appreciable abundance. In this realm, the structure of the anesthetic haloether

 Please wait while we load your content...
Please wait while we load your content...