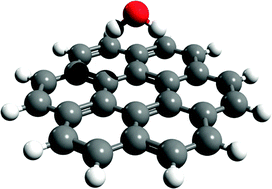
Benchmark calculations of water–acene interaction energies: Extrapolation to the water–graphene limit and assessment of dispersion–corrected DFT methods
Abstract
In a previous study (J. Phys. Chem.
- This article is part of the themed collection: Characterization of adsorbed species

 Please wait while we load your content...
Please wait while we load your content...