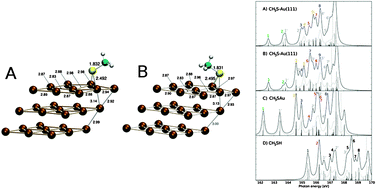
The relativistic four-component static exchange approach for calculation of near-edge X-ray absorption spectra has been reviewed. Application of the method is made to the Au(111) interface and the adsorption of methanethiol by a study of the near sulfur L-edge spectrum. The binding energies of the sulfur 2p1/2 and 2p3/2 sublevels in methanethiol are determined to be split by 1.2 eV due to spin–orbit coupling, and the binding energy of the 2p3/2 shell is lowered from 169.2 eV for the isolated system to 167.4 and 166.7–166.8 eV for methanethiol in mono- and di-coordinated adsorption sites, respectively (with reference to vacuum). In the near L-edge X-ray absorption fine structure spectrum only the σ*(S–C) peak at 166 eV remains intact by surface adsorption, whereas transitions of predominantly Rydberg character are largely quenched in the surface spectra. The σ*(S–H) peak of methanethiol is replaced by low-lying, isolated, σ*(S–Au) peak(s), where the number of peaks in the latter category and their splittings are characteristic of the local bonding situation of the sulfur.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...