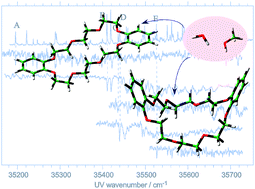
The conformation and complex formation with guest molecules have been investigated for jet-cooled dibenzo-24-crown-8-ether (DB24C8) by laser induced fluorescence (LIF), UV-UV hole-burning (UV-UV HB), and IR-UV double-resonance (IR-UV DR) spectroscopy. The results are compared with the results of dibenzo-18-crown-6-ether (DB18C6) and analyzed by density functional theory (DFT) calculations at the B3LYP/6-31+G* level. Five conformers are identified for DB24C8 under jet-cooled conditions, and the structure of the major isomer is determined to be a boat form, similar to the case of DB18C6. Two molecules (water and methanol) are investigated as the guest species for the encapsulation experiment. DB24C8 hardly encapsulates a water molecule, different from the case of DB18C6 in which a water molecule is efficiently captured. However, it is likely that larger (water)n clusters can be captured in the DB24C8 cavity. The different feature in the complex formation between DB24C8 and DB24C8 with the water molecules are attributed to a larger cavity size of DB24C8 than that of DB18C6. For methanol, two kinds of DB18C6–(methanol)1 isomers are identified by IR-UV DR spectroscopy; one is the “OH⋯π” H-bonded isomer, characteristic of methanol with one OH group, and the other is the “OH⋯O” H-bonded ones. These results indicate the multiple H-bond formation to the oxygen atoms of the ether ring may play an important role in the complexes of DB24C8 or DB18C6 with water.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...