Ionicity in ionic liquids: correlation with ionic structure and physicochemical properties
Abstract
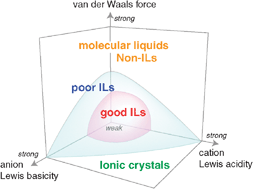
Ionic liquids (ILs) are ambient temperature molten salts that have attracted considerable attention because of their negligible volatility, thermal stability, nonflammability, and high ionic conductivity. These remarkable properties result essentially from their ionic nature. Thus, the concept of ionicity of ILs (i.e. how ionic they are) is of great significance for characterising their properties. Here, we show the methodologies used to assess the ionic nature in ILs. On the basis of quantitative estimation of the ionicity, their dependence on ionic structure, polarity scales, and physicochemical properties is reviewed. The ionicity of certain ILs is also predicted from their physicochemical properties. The effects of different classes of ILs (e.g., protic ILs and lithium ILs) and binary systems consisting of ILs and other components on the ionicity are also discussed.
- This article is part of the themed collection: Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...