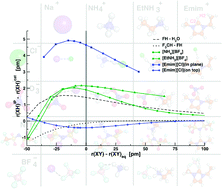
The intermediate bond forces in ionic liquids are investigated from static quantum chemical calculations at various methods and two basis sets. The experimentally observed red-shift of the donor-proton bond stretching frequency due to a bond elongation is confirmed by all methods. Comparing Hartree–Fock to second-order Møller–Plesset perturbation theory, the Hartree–Fock method gives in many cases an erroneous description of the geometries. Furthermore, the Hartree–Fock interaction energies can deviate up to 60 kJ mol−1 from Møller–Plesset perturbation theory indicating the importance of dispersion interaction. While the usual trends of decreasing stability or interaction energies with increasing ion sizes are found, the geometries involving hydrogen atoms do not change this order of total interaction energies. Therefore, the hydrogen bond is not the most important interaction for ion pairs with regard to the total interaction energy. On the other hand, the different established analysis methods give rise to hydrogen bonding in several ion pairs. Charge analysis reveals the hydrogen-bonding character of the ion pair and shows, depending on the type of ions combined and further on the type of conformers considered, that a hydrogen bond can be present. The possibility of hydrogen bonding is also shown by an analysis of the frontier orbitals. Calculating potential energy surfaces and observing from this the change in the donor proton bond indicates that regular hydrogen bonds are possible in ion pairs of ionic liquids. Thereby, the maximum of bond elongation exceeds the one of a usual hydrogen bond by far. The more salt-like hydrogen-bonded ion pair [NH4][BF4] exhibits a steeper maximum than the more ionic liquid like ion pair [EtNH3][BF4]. The fact that imidazolium-based ionic liquids as [Emim][Cl] can display two faces, hydrogen bonding and purely ionic bonding, points to a disturbing rather than stabilizing role of hydrogen bonding on the interaction of the counterions in imidazolium-based ionic liquids. While geometry and charge analysis provides attributes of weak (blue-shifted) hydrogen bonds, large bond elongations accompanied by red-shifts are obtained for the ion pairs investigated. This can be understood by the simple fact that these imidazolium-based ionic liquid ion pairs constitute weak hydrogen bonds placed between two delocalized charges.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...