Internal mobilities and diffusion in an ionic liquid mixture
Abstract
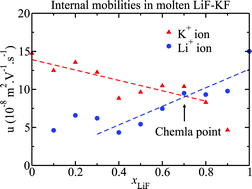
The internal mobility gives the rate at which one ionic species moves relative to the other species present in an ionic mixture, it mirrors the differential strength of the interactions between different ionic species. In this work we examine the dependence of the internal mobilities of the

 Please wait while we load your content...
Please wait while we load your content...