A detailed knowledge of the capsid assembly pathways of viruses from their coat protein building blocks is required to devise novel therapeutic strategies to inhibit such assembly. In the quest for understanding how assembly of single-stranded RNA viruses is achieved at the molecular level, HDX-MS has been used to locate regions of a coat protein dimer that exhibit conformational/dynamical changes, and hence changes in their HDX kinetics, upon binding to a genomic RNA stem-loop known to trigger assembly initiation. The HDX-MS data highlight specific areas within the coat protein dimer that alter their exchange kinetics in the presence of the RNA. These include the known RNA-binding sites, β-strands E and G, which have a lower susceptibility to HDX when ligand-bound, as may have been expected. In contrast, several exposed regions are unaffected by ligand binding. Significantly in this example, the loop between β-strands F and G exhibits reduced HDX propensity when the RNA is bound, consistent with previous inferences from NMR and normal mode analysis that suggested a local conformational change at this loop induced by dynamic allostery. These results demonstrate the potential utility of HDX to probe conformational and dynamical changes within non-covalently bound protein–ligand complexes which are of widespread importance in many biomolecular systems.
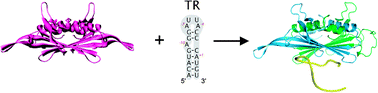
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
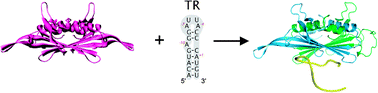

 Please wait while we load your content...
Please wait while we load your content...