CCl4 dissociation on the ice Ih surface: an excess electron mediated process
Abstract
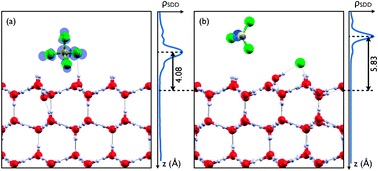
Dissociation of chlorofluorocarbons in the atmosphere is a heterogeneous process that takes place mainly on the surface of ice particles. Recently an enhancement of the dissociation rate due to excess electrons has been shown theoretically and correspondingly measured experimentally. Our density functional theory calculations show that
 Please wait while we load your content...
Please wait while we load your content...