4-Ethynylpyridine and 5-ethynylpyrimidine were utilized as ligands to synthesize three novel silver–ethynide complexes [(4-Py-C2Ag)3·6CF3CO2Ag·2H2O] (1), [(4-Py-C2Ag)2·4AgNO3] (2) and [(5-Pyrim-C2Ag)·3AgNO3·H2O] (3). In both 1 and 2, the [4-C5H4N(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)Ag4]3+ units aggregate to generate linear silver chains through silver–ethynide and argentophilic interactions, which are further connected through Ag–N(pyridyl) coordination bonds to afford 2D grids, and finally linked by trifluoroacetate or nitrate groups to form a rigid 3D coordination network. However in 3, each [5-C4H3N2(C
C)Ag4]3+ units aggregate to generate linear silver chains through silver–ethynide and argentophilic interactions, which are further connected through Ag–N(pyridyl) coordination bonds to afford 2D grids, and finally linked by trifluoroacetate or nitrate groups to form a rigid 3D coordination network. However in 3, each [5-C4H3N2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)Ag4]3+ unit interacts with two other units through Ag–N(pyrimidyl) bonds to construct 2D honeycomb-like networks, and are also further connected by nitrate groups to generate a 3D network. It is demonstrated that the coordination modes of organic ligands play a key role in controlling the structures of the organometallic networks based on silver–ethynide complexes.
C)Ag4]3+ unit interacts with two other units through Ag–N(pyrimidyl) bonds to construct 2D honeycomb-like networks, and are also further connected by nitrate groups to generate a 3D network. It is demonstrated that the coordination modes of organic ligands play a key role in controlling the structures of the organometallic networks based on silver–ethynide complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)Ag4]3+ units aggregate to generate linear
C)Ag4]3+ units aggregate to generate linear ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)Ag4]3+ unit interacts with two other units through
C)Ag4]3+ unit interacts with two other units through 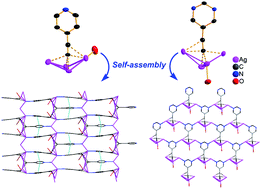

 Please wait while we load your content...
Please wait while we load your content...