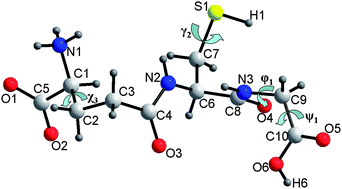
The hydrostatic compression of glutathione (GSH) has been studied to 5.24 GPa by single crystal X-ray diffraction. Over the course of this pressure range the compound undergoes two phase transitions, the first between 1.65 and 2.27 GPa, yielding GSH-II, and the second between 2.94 and 3.70 GPa, yielding GSH-III. All three polymorphs are orthorhombic, P212121, and feature layers of GSH molecules. These layers are connected together via alternating layers of R33(11) motifs, and OH⋯O and one NH⋯O H-bonding interactions. In the phase-I to II transition voids at the centre of ring motifs begin to close-up, accompanied by two significant changes in the conformation of the GSH molecules. The first is a large re-orientation of the glycine residue quantified by one of the CNCC torsion angles along the backbone of the peptide which increases from ca. −76 to 117°. The second involves a re-orientation of the OH⋯O interaction, which breaks the aforementioned H-bond which acted between layers, to form a much shorter H-bonding interaction in GSH-II within the layers. The conformation of this interaction is rather unfavourable under ambient pressure conditions, however, is stabilised in the solid state structure of GSH-II, shown here by mapping the potential energy surface of the CCOH torsion angle. It is speculated here that the closure of voids within ring motifs, and formation of this shorter OH⋯O interaction are the main driving forces for this transition. On increasing pressure further, formation of GSH-III at 3.70 GPa involves three main structural changes: (i) a further twisting of the glycine residue, quantified by a CNCC torsion angle decrease from ca. 117° to 69°; (ii) a twisting along the glutamic acid residue backbone, quantified by a CCCN torsion angle increase from ca. −79° to −73°; (iii) a change in orientation of the thiol group, quantified by a CCSH torsion angle increase from 83° to 149°. The effect of (i) and (ii) is to increase the effective length of the GSH molecule backbone resulting in an increase in length of the b-axis. This allows the layers to pack more efficiently and further closures of voids within ring motifs can be observed. The conformational change observed in (ii) also allows the formation of a much shorter SH⋯O H-bonding interaction resulting in the conformational change observed in (iii). It is postulated here that both more efficient packing of the layers and formation of a shorter SH⋯O interaction are the driving forces for the phase-II to III transition observed here.

 Please wait while we load your content...
Please wait while we load your content...