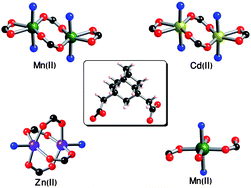
A series of flexible metal–organic frameworks (MOFs) have been successfully synthesized under hydrothermal condition using 1,3-adamantanediacetic acid (C14H20O4, H2ADA) as a flexible dicarboxylate building block, 4,4′-bipyridine and transition metal ions [Cd(II), Zn(II), and Mn(II)] as metal centers in DMF and aqueous media. These MOFs formulated as [Cd(ADA)(4,4′-bipy)0.5]·(DMF) (Cd-ADA-1), [Mn(ADA)(4,4′-bipy)0.5]·(DMF) (Mn-ADA-1), Zn(ADA)(4,4′-bipy)0.5 (Zn-ADA-1), and [Mn(HADA)2(4,4′-bipy)(H2O)2] (Mn-ADA-2) (ADA = 1,3-adamantanediacetate, 4,4′-bipy = 4,4′-bipyridine and DMF = N,N′-dimethyl formamide) display interesting 1D, 2D and 3D structural features depending on the solvent of synthesis. All these MOFs were structurally determined by single-crystal X-ray diffraction. The coordination modes of this ligand are discussed and in addition, thermal stability and hydrogen (H2) and carbon-dioxide (CO2) adsorption properties of Cd-ADA-1, Mn-ADA-1 and Zn-ADA-1 are also presented. Hydrogen sorption at 77 K and up to 1 atm is found to be 0.42, 0.72 and 1.36 wt% without saturation for Zn-ADA-1, Mn-ADA-1 and Cd-ADA-1 samples.

 Please wait while we load your content...
Please wait while we load your content...