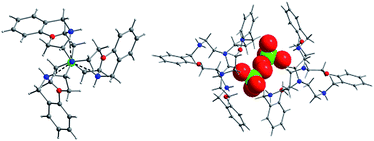
The X-ray crystallographic study of various anionic complexes have been performed on two laterally non-symmetric aza-oxa cryptands Lo and Lm having different cavity dimension. The anions are bound through various N/C/O–H⋯X (X = anion) bonding interactions with the cryptand receptors. For the chloride complex of Lo (complex 1), the encapsulated chloride resides perfectly on C3 axis and prefers to stay at the ‘oxa’ end despite H- bonding interactions with protonated ‘aza’ N atoms. In case of the perchlorate (complex 2), the anion remains outside the cavity. The anions occupy the cavity created by four arms of two cryptand molecules and also the space provided by the two arms of a single cryptand molecule. Although, Lm has a larger cavity, perchlorate still remains outside (complex 3). To probe preferences for encapsulation, cryptand Lm is allowed to react with three different binary mixtures of acids. For the case of HCl–H2SO4 and HCl-HNO3 binary systems (complex 4 and 5, respectively), Cl− is incorporated in the cavity leaving SO4− or NO3− outside. In case of HBr-HNO3 mixture, NO3− is preferred over Br− in the cavity (complex 6). In each case for Lm, the encapsulated anion is displaced away from ‘oxa’ end to the ‘aza’ end of the cavity. Interestingly, in 2–6 the secondary amino N in the bridges and the bridgehead N at the ‘aza’ end gets protonated. In these compounds, water clusters of various nuclearity have been identified.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...