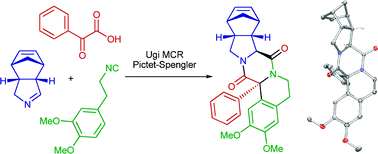
Asymmetric synthesis of synthetic alkaloids by a tandem biocatalysis/Ugi/Pictet–Spengler-type cyclization sequence†
Abstract
We have combined the biocatalytic desymmetrization of 3,4-cis-substituted meso-pyrrolidines with an Ugi-type multicomponent reaction followed in situ by a Pictet–Spengler-type

 Please wait while we load your content...
Please wait while we load your content...