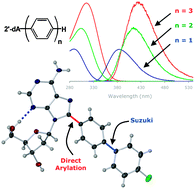
A sequential direct arylation/Suzuki–Miyaura cross-coupling transformation of unprotected 2′-deoxyadenosine affords a novel class of fluorescent analogues†
Abstract
Novel rigid 8-biaryl-2′-deoxyadenosines with tuneable fluorescent properties can be accessed by an efficient sequential

 Please wait while we load your content...
Please wait while we load your content...