Well-defined and easily accessible yttrium complexes for enantioselective cyclisation of amines tethered to 1,2-di-substituted alkenes†
Abstract
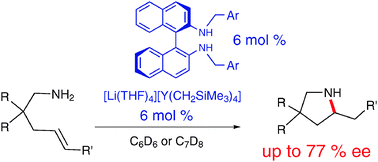
A straightforward in situ preparation of new chiral amido alkyl ate yttrium complexes is described. They catalysed the enantioselective

 Please wait while we load your content...
Please wait while we load your content...