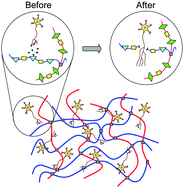
A key attribute missing from many current biomaterials is the ability to independently tune multiple biomaterial properties without simultaneously affecting other material parameters. Because cells are well known to respond to changes in the initial elastic modulus, degradation rate, and cell adhesivity of a biomaterial, it is critical to develop synthetic design strategies that allow decoupled tailoring of each individual parameter in order to systematically optimize cell-scaffold interactions. We present the development of a biomimetic scaffold composed of chemically crosslinked, elastin-like proteins designed to support neural regeneration through a combination of cell adhesion and cell-induced degradation and remodeling. The design of these engineered proteins includes cell adhesion sequences to enable neuronal attachment as well as sequences sensitive to cleavage by urokinase plasminogen activator (uPA), a protease locally secreted from the tips of growing neurites, to enable highly localized and tunable degradation properties. These engineered proteins are produced using recombinant techniques and chemically crosslinked into highly swollen hydrogels with controllable mechanical properties. Through a modest 3% change in the chemical identity of three otherwise identical engineered proteins, we can modify the uPA substrate specificity resulting in tunable changes in protease degradation half-life over two orders of magnitude. Under high uPA exposure, the designed scaffolds exhibit systematic variation of scaffold lifetime, from being fully degraded within a single day to showing no noticeable degradation within a full week. In vitro studies using the model PC-12 neuronal-like cell line show that the crosslinked proteins support tunable cell adhesion and neuronal differentiation. Increasing the density of RGD peptides present in the protein substrates leads to increased cell adhesion and more extensive neurite outgrowth. These engineered proteins offer the ability to independently tailor the mechanics, degradation properties, and cell adhesivity of scaffolds for the study of central nervous system regeneration.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...