Time-resolved methods in biophysics. 10. Time-resolved FT-IR difference spectroscopy and the application to membraneproteins†
Abstract
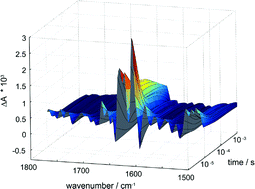
The introduction of time-resolved
- This article is part of the themed collection: Time-resolved methods in biophysics series

 Please wait while we load your content...
Please wait while we load your content...