Demetalation kinetics of natural chlorophylls purified from oxygenic photosynthetic organisms: effect of the formyl groups conjugated directly to the chlorin π-macrocycle
Abstract
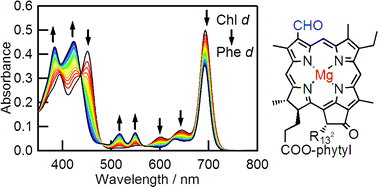
Demetalation kinetics of natural chlorophyll (Chl) d purified from Acaryochloris marina was first studied and compared with those of Chls a and b. The demetalation rate constant of Chl d, which possessed a formyl group at the 3-position, was five-fold smaller than that of Chl a possessing a vinyl group at the same position in aqueous acetone at the proton concentration of 1.2 × 10−3 M at 25 °C. In contrast, the demetalation rate constant of Chl b possessing a formyl group at the 7-position was 26 times smaller than that of Chl a. The activation energy of demetalation reaction of Chl d was larger than that of Chl a, but smaller than that of Chl b. These indicate that the substitution effect of 3-formyl group on the acidic removal of central magnesium in Chls was smaller than that of 7-formyl group.

 Please wait while we load your content...
Please wait while we load your content...